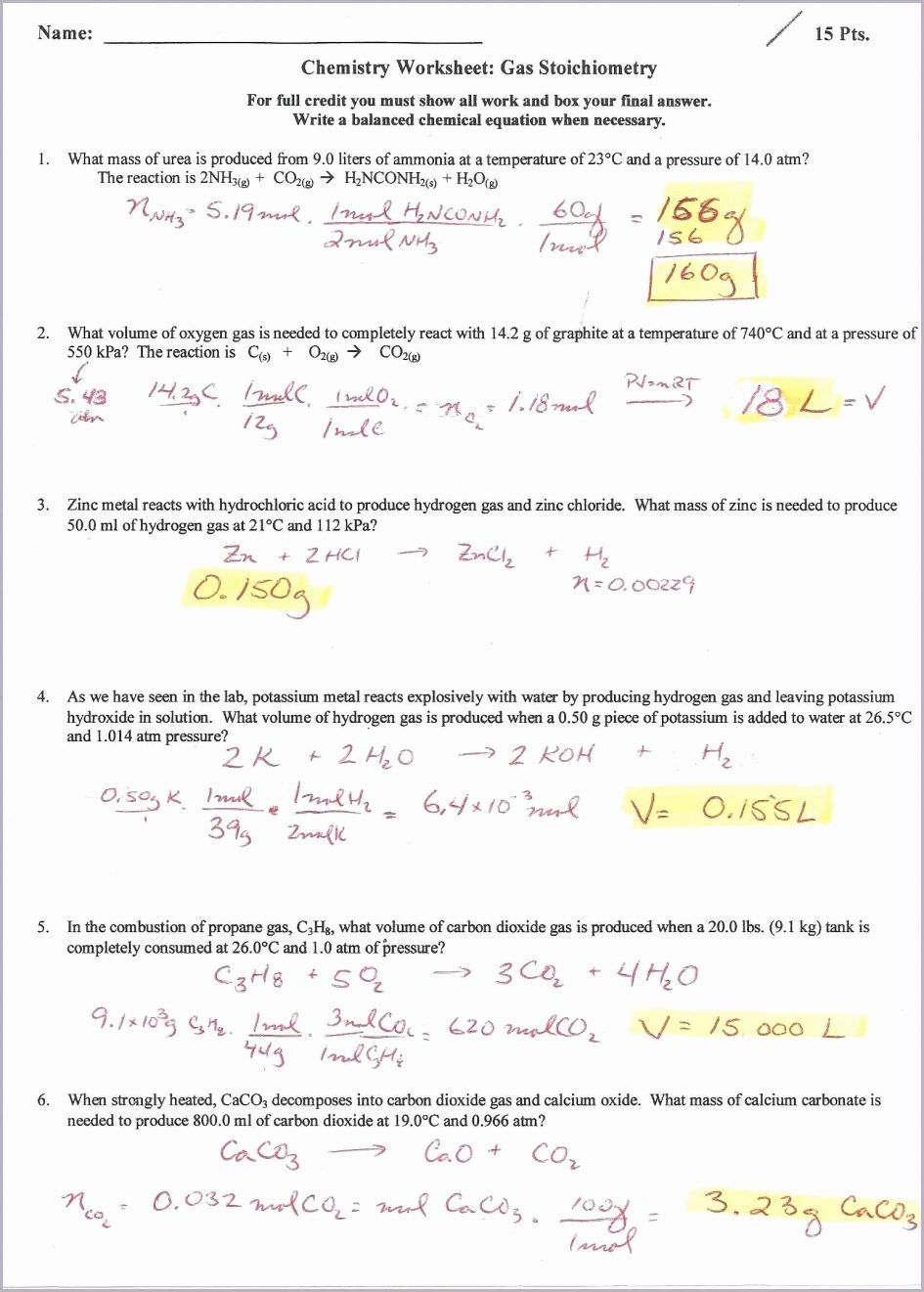
Studying the ideal gas law is essential in understanding the behavior of gases under different conditions. A worksheet that focuses on this law can help students practice and reinforce their knowledge of the concept. By working through problems and calculations, students can gain a better grasp of how pressure, volume, temperature, and the number of moles of a gas are related.
When students are given an ideal gas law worksheet, they are typically presented with a series of problems that require them to apply the formula PV = nRT. This formula expresses the relationship between the pressure (P), volume (V), number of moles (n), gas constant (R), and temperature (T) of a gas. By solving these problems, students can see firsthand how changes in one variable can affect the others.
One common type of problem on an ideal gas law worksheet involves calculating the final pressure, volume, or temperature of a gas when one or more of these variables are changed. Students may need to rearrange the formula to solve for the unknown variable, which requires critical thinking and problem-solving skills. These types of problems help students develop a deeper understanding of the principles behind the ideal gas law.
Another aspect of an ideal gas law worksheet may involve using the concept of stoichiometry to determine the amount of reactants or products in a chemical reaction. By applying the ideal gas law to these types of problems, students can see how gases behave in real-world scenarios and gain a more practical understanding of the concept.
In conclusion, an ideal gas law worksheet is a valuable tool for students to practice and reinforce their understanding of the relationships between pressure, volume, temperature, and the number of moles of a gas. By working through problems and calculations, students can improve their problem-solving skills and gain a deeper understanding of the principles behind the ideal gas law. These worksheets are essential for mastering the concept and preparing for exams or assessments.