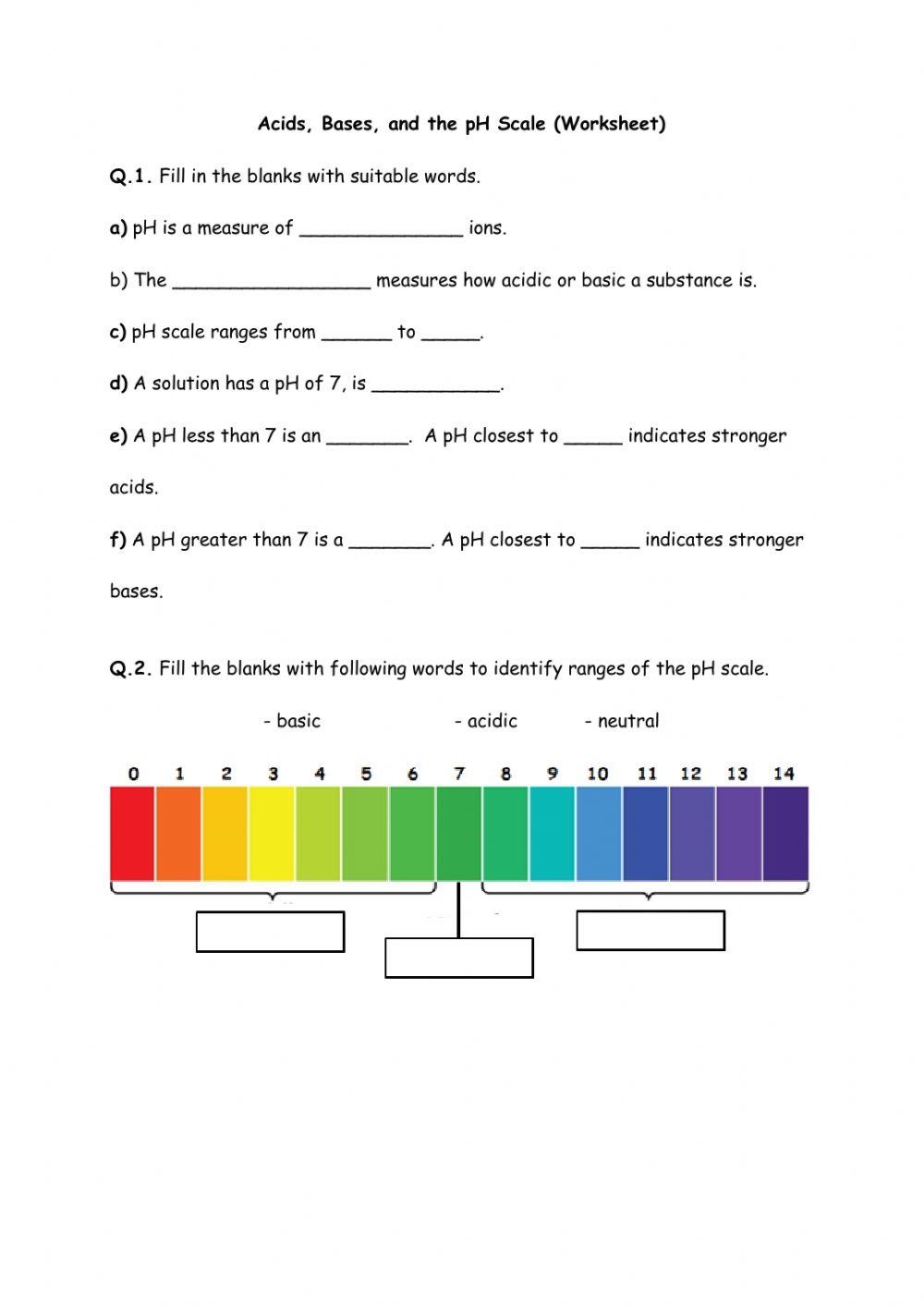
Understanding pH is essential in chemistry as it measures the acidity or alkalinity of a solution. pH is a scale from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are alkaline. pH worksheets are effective tools for students to practice calculating pH levels of various solutions.
Students can use pH worksheets to determine the pH of common substances such as lemon juice, vinegar, and baking soda. By following the steps on the worksheet, students can calculate the hydrogen ion concentration in the solution and convert it to pH using the formula pH = -log[H+]. This hands-on practice helps students grasp the concept of pH and its importance in everyday life.
Furthermore, pH worksheets can include scenarios where students have to identify whether a solution is acidic, alkaline, or neutral based on its pH value. By analyzing different pH levels, students can develop critical thinking skills and understand the impact of pH on the environment, human health, and industrial processes.
Another aspect of pH worksheets is titration problems, where students have to calculate the pH of a solution after adding a specific amount of acid or base. This exercise helps students understand the concept of titration and how it can be used to determine the unknown concentration of a solution. pH worksheets with titration problems enhance students’ problem-solving abilities and prepare them for more advanced chemistry concepts.
In conclusion, pH worksheets are valuable tools for students to practice calculating pH levels, identifying acidic or alkaline solutions, and solving titration problems. By engaging in these exercises, students can enhance their understanding of pH and its significance in chemistry. Incorporating pH worksheets in the curriculum can make learning about pH more interactive and enjoyable for students.